Europa Ultra NC
Non-Compliant Balloon Catheter
” The superior high force for the demanding Interventionalist ”
Low profile, high pressure angioplasty catheters designed to be used in dilating the unyielding fibrocalcified lesions with a high pressure application providing controlled expansion, as well.
The use of Europa Ultra NCTM for post-dilatation of DES/or BMS optimizes stent-to-wall apposition, reduces edge effects and minimizes vessel wall damage post stenting, even in stiff fibrotic lesions
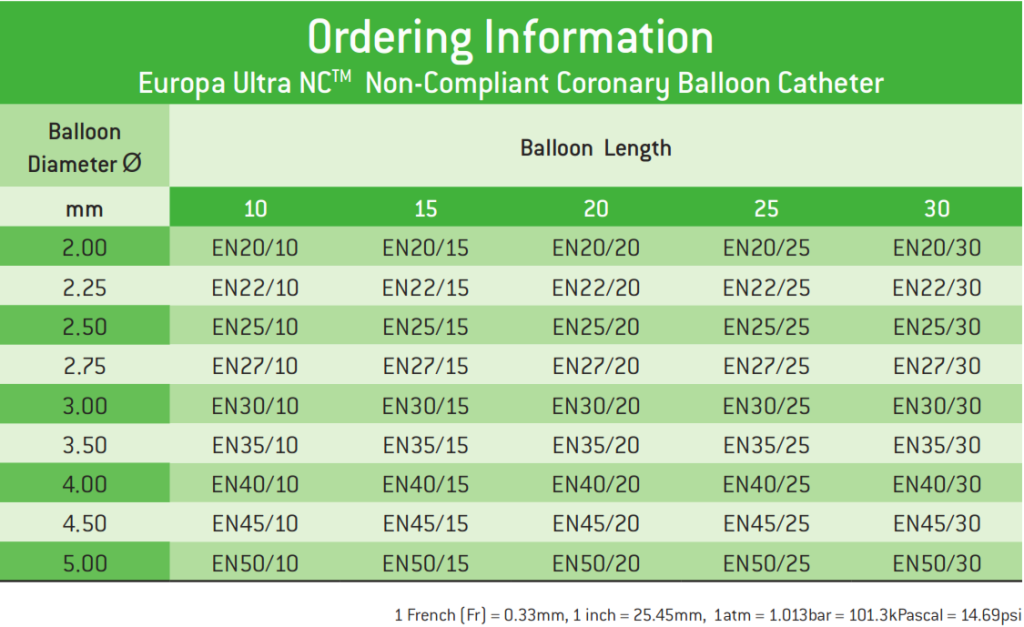
Europa Ultra NCTM Non-Compliant Coronary Balloon Catheter
Mode of exchange: Rx
Baloon material / characteristic: modified polyamide / non-compliant
Guiding Catheter compatibility (min): 5F
Usable Catheter length: 140 cm
Radiopaque markers: 2, Pt-Ir
Distal shaft: 2.4F (Ø 2.00 mm)
2.5F (Ø 2.25 – 3.50 mm)
2.9F (Ø 4.00 – 5.00 mm)
Hypotube: 2.0F
Guidewire compatibility (max): 0.014”
Nomimal pressure: 10 atm
Rated Burst Pressure: 18 – 20 atm
Coating: Hydrophilic, HiFlow
Total shelf life: 4 years
Improved trackability
- Non-Compliant design, resisting deformation when subjected to pressure
- Controlled minimal balloon growth (5%) between NP & RBP for flat compliance
- High pressure catheter (RBP up to 20 atm)
- Optimal accuracy is ensured even under high dilatation forces while maintaining dimensional stability
- Ultra-low 0.016’’ tip profile
- Soft, short and tapered atraumatic tip
- Rontis’ proprietary balloon material blend ensures decreased wall thickness
- Rontis’ proprietary hydrophilic coating, HiFlow for enhanced lubricity
- Homogeneous balloon expansion
- Tri-folding balloon technology